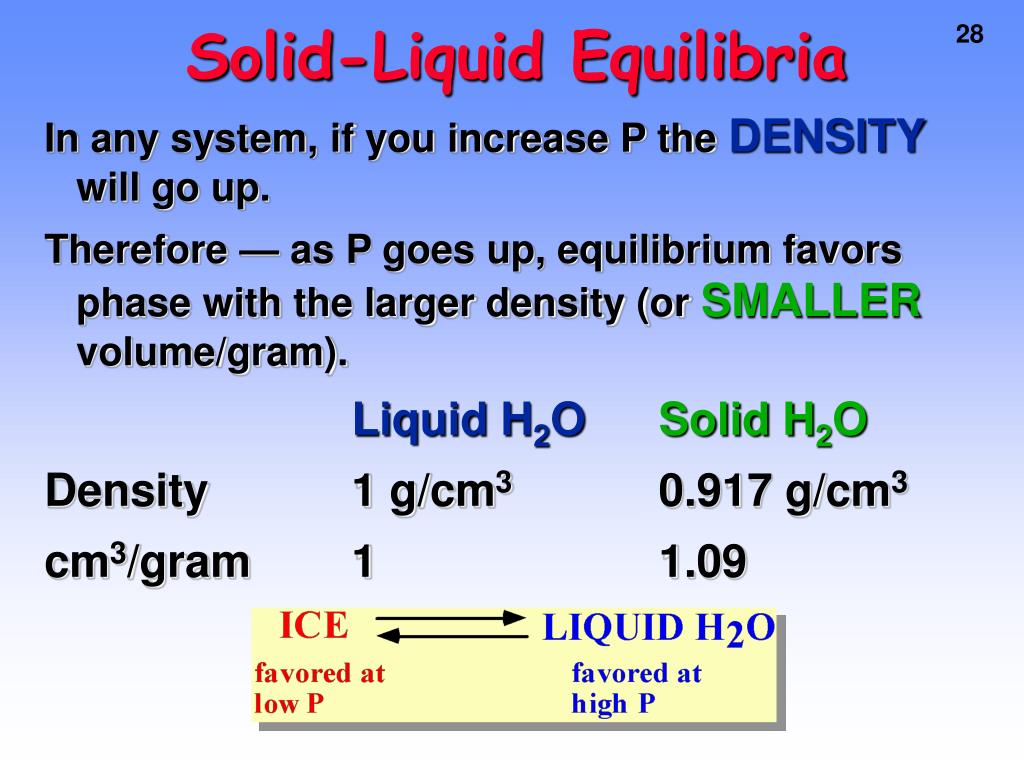
Solid Liquid Equilibrium . A state where both solid and liquid phases coexist in equilibrium at a specific temperature and. equation 12.5.25 is a general equation that applies even if the solution saturated with one salt contains a second salt with a common ion. The method of thermal analysis is used to. What happens if you keep ice and water in a perfectly insulated manner, such as in a thermos flask at a.
from www.slideserve.com
equation 12.5.25 is a general equation that applies even if the solution saturated with one salt contains a second salt with a common ion. The method of thermal analysis is used to. What happens if you keep ice and water in a perfectly insulated manner, such as in a thermos flask at a. A state where both solid and liquid phases coexist in equilibrium at a specific temperature and.
PPT Liquids PowerPoint Presentation, free download ID355773
Solid Liquid Equilibrium The method of thermal analysis is used to. A state where both solid and liquid phases coexist in equilibrium at a specific temperature and. equation 12.5.25 is a general equation that applies even if the solution saturated with one salt contains a second salt with a common ion. The method of thermal analysis is used to. What happens if you keep ice and water in a perfectly insulated manner, such as in a thermos flask at a.
From learncheme.com
solidsolidliquidphasediagramsconceptestandexampleproblem Solid Liquid Equilibrium The method of thermal analysis is used to. A state where both solid and liquid phases coexist in equilibrium at a specific temperature and. equation 12.5.25 is a general equation that applies even if the solution saturated with one salt contains a second salt with a common ion. What happens if you keep ice and water in a perfectly. Solid Liquid Equilibrium.
From unistudium.unipg.it
Phase Diagrams Solid Liquid Equilibrium A state where both solid and liquid phases coexist in equilibrium at a specific temperature and. equation 12.5.25 is a general equation that applies even if the solution saturated with one salt contains a second salt with a common ion. The method of thermal analysis is used to. What happens if you keep ice and water in a perfectly. Solid Liquid Equilibrium.
From chemistry.stackexchange.com
phase Thermodynamics of SolidLiquid Chemistry Stack Exchange Solid Liquid Equilibrium equation 12.5.25 is a general equation that applies even if the solution saturated with one salt contains a second salt with a common ion. A state where both solid and liquid phases coexist in equilibrium at a specific temperature and. What happens if you keep ice and water in a perfectly insulated manner, such as in a thermos flask. Solid Liquid Equilibrium.
From courses.lumenlearning.com
Phase Diagrams Chemistry Solid Liquid Equilibrium What happens if you keep ice and water in a perfectly insulated manner, such as in a thermos flask at a. A state where both solid and liquid phases coexist in equilibrium at a specific temperature and. The method of thermal analysis is used to. equation 12.5.25 is a general equation that applies even if the solution saturated with. Solid Liquid Equilibrium.
From www.researchgate.net
Equilibrium concentrations of the solid and the liquid phase for the Solid Liquid Equilibrium What happens if you keep ice and water in a perfectly insulated manner, such as in a thermos flask at a. The method of thermal analysis is used to. equation 12.5.25 is a general equation that applies even if the solution saturated with one salt contains a second salt with a common ion. A state where both solid and. Solid Liquid Equilibrium.
From chem.libretexts.org
12.5 SolidLiquid Equilibria Chemistry LibreTexts Solid Liquid Equilibrium A state where both solid and liquid phases coexist in equilibrium at a specific temperature and. What happens if you keep ice and water in a perfectly insulated manner, such as in a thermos flask at a. The method of thermal analysis is used to. equation 12.5.25 is a general equation that applies even if the solution saturated with. Solid Liquid Equilibrium.
From www.tf.uni-kiel.de
Liquid solid phase diagrams Solid Liquid Equilibrium What happens if you keep ice and water in a perfectly insulated manner, such as in a thermos flask at a. The method of thermal analysis is used to. A state where both solid and liquid phases coexist in equilibrium at a specific temperature and. equation 12.5.25 is a general equation that applies even if the solution saturated with. Solid Liquid Equilibrium.
From www.slideserve.com
PPT Phase Equilibrium PowerPoint Presentation, free download ID2503286 Solid Liquid Equilibrium What happens if you keep ice and water in a perfectly insulated manner, such as in a thermos flask at a. The method of thermal analysis is used to. equation 12.5.25 is a general equation that applies even if the solution saturated with one salt contains a second salt with a common ion. A state where both solid and. Solid Liquid Equilibrium.
From slidetodoc.com
Phase Diagrams 1 2 TRANSITIONS BETWEEN PHASES Section Solid Liquid Equilibrium equation 12.5.25 is a general equation that applies even if the solution saturated with one salt contains a second salt with a common ion. What happens if you keep ice and water in a perfectly insulated manner, such as in a thermos flask at a. The method of thermal analysis is used to. A state where both solid and. Solid Liquid Equilibrium.
From chem.libretexts.org
12.5 SolidLiquid Equilibria Chemistry LibreTexts Solid Liquid Equilibrium equation 12.5.25 is a general equation that applies even if the solution saturated with one salt contains a second salt with a common ion. A state where both solid and liquid phases coexist in equilibrium at a specific temperature and. What happens if you keep ice and water in a perfectly insulated manner, such as in a thermos flask. Solid Liquid Equilibrium.
From www.researchgate.net
Polythermal solidliquid equilibrium diagram of the system Li 2 SO 4 Solid Liquid Equilibrium A state where both solid and liquid phases coexist in equilibrium at a specific temperature and. What happens if you keep ice and water in a perfectly insulated manner, such as in a thermos flask at a. equation 12.5.25 is a general equation that applies even if the solution saturated with one salt contains a second salt with a. Solid Liquid Equilibrium.
From learncheme.com
ssleequilibrium LearnChemE Solid Liquid Equilibrium equation 12.5.25 is a general equation that applies even if the solution saturated with one salt contains a second salt with a common ion. What happens if you keep ice and water in a perfectly insulated manner, such as in a thermos flask at a. A state where both solid and liquid phases coexist in equilibrium at a specific. Solid Liquid Equilibrium.
From cepdlccl.blob.core.windows.net
Solid Liquid Equilibrium Example at Timothy Bolander blog Solid Liquid Equilibrium equation 12.5.25 is a general equation that applies even if the solution saturated with one salt contains a second salt with a common ion. What happens if you keep ice and water in a perfectly insulated manner, such as in a thermos flask at a. The method of thermal analysis is used to. A state where both solid and. Solid Liquid Equilibrium.
From www.scribd.com
Solid Liquid Equilibrium Phase (Matter) Phase Diagram Solid Liquid Equilibrium What happens if you keep ice and water in a perfectly insulated manner, such as in a thermos flask at a. equation 12.5.25 is a general equation that applies even if the solution saturated with one salt contains a second salt with a common ion. A state where both solid and liquid phases coexist in equilibrium at a specific. Solid Liquid Equilibrium.
From cepdlccl.blob.core.windows.net
Solid Liquid Equilibrium Example at Timothy Bolander blog Solid Liquid Equilibrium What happens if you keep ice and water in a perfectly insulated manner, such as in a thermos flask at a. A state where both solid and liquid phases coexist in equilibrium at a specific temperature and. The method of thermal analysis is used to. equation 12.5.25 is a general equation that applies even if the solution saturated with. Solid Liquid Equilibrium.
From dbcarnahanoutmantles.z21.web.core.windows.net
Phase Diagrams Understanding The Basics Solid Liquid Equilibrium The method of thermal analysis is used to. What happens if you keep ice and water in a perfectly insulated manner, such as in a thermos flask at a. equation 12.5.25 is a general equation that applies even if the solution saturated with one salt contains a second salt with a common ion. A state where both solid and. Solid Liquid Equilibrium.
From slideplayer.com
Phase Changes. ppt download Solid Liquid Equilibrium The method of thermal analysis is used to. A state where both solid and liquid phases coexist in equilibrium at a specific temperature and. equation 12.5.25 is a general equation that applies even if the solution saturated with one salt contains a second salt with a common ion. What happens if you keep ice and water in a perfectly. Solid Liquid Equilibrium.
From www.slideserve.com
PPT Liquids, solids, & intermolecular forces PowerPoint Presentation Solid Liquid Equilibrium The method of thermal analysis is used to. What happens if you keep ice and water in a perfectly insulated manner, such as in a thermos flask at a. A state where both solid and liquid phases coexist in equilibrium at a specific temperature and. equation 12.5.25 is a general equation that applies even if the solution saturated with. Solid Liquid Equilibrium.